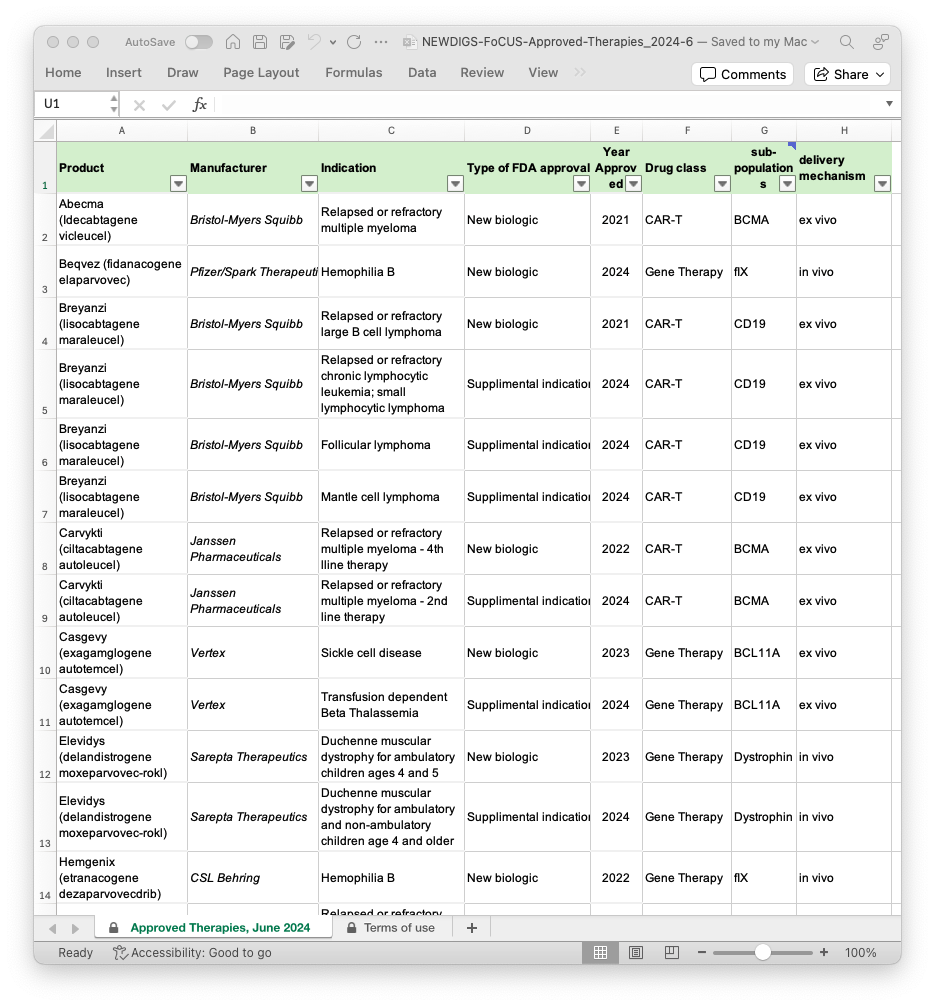
Approved cell and gene therapy (CGT) products
NEWDIGS maintains a list of approved durable cell and gene therapies (CGT), including approval dates for new biologics as well as supplemental indications. Durable cell and gene products are designed for one-time use, as compared to products which are administered more than once.
All therapies on this list have gone through the FDA’s drug approval process, which is designed to ensure that drugs are safe and effective for their intended use before they are made available to the public. Within the FDA, the Center for Biologics Evaluation and Research (CBER) reviews and regulates cell therapy products, human gene therapy products, and devices related to cell and gene therapy. CBER uses both the Public Health Service Act and the Federal Food Drug and Cosmetic Act as enabling statutes for oversight.
CGTs aim to treat or prevent diseases by introducing, removing, or altering genetic material within a patient's cells. As a disease may be caused by various damaged, missing, or mutated genes, our list identifies the genes targeted by each approved product. the gene targeted by the approved product is also identified on our list and can serve as a reference for your use of the Individual Indication workbook.
To consider the impact of approved CGT, it is also helpful to understand the delivery approaches as these impact treatment time, cost, and therapy targeting. The two main approaches for delivering the therapies differ in where the modification happens: inside the body (in vivo) or outside the body (ex vivo).
Ex vivo CGT therapy takes cells from a patient’s body and genetically modifies them in a lab before returning them to the body. The lab setting allows scientists to confirm that the newly introduced genetic material is functioning as intended before reintroducing the genetic material to the body.
- Ex vivo therapy methods include CAR-T and ex vivo gene transfer.
- Ex vivo therapies are most commonly used to target cells that are not easily accessible or in cases with serious concern about a patient’s immune response to treatment. For these reasons, ex vivo therapies are typically used to treat blood disorders and diseases, and external organs like the eyes.
In vivo CGT therapy, new genetic material is created in a lab and then delivered into a patient’s body directly by infusing the material into the blood or injecting it directly into the target organ.
- In vivo therapy methods include the use of viral vectors (modified viruses that have been stripped of their disease-causing abilities and use viruses or other methods to deliver genes directly into a patient’s cells) such as CRISPR-Cas9 therapy, RNA Interference (RNAi), and naked DNA injection.
- In vivo gene therapies are often used to treat genetic disorders that affects one specific gene or to target internal organs such as the heart, brain, or lungs.
Key differences
|
Process complexity |
|
|
Ex vivo therapy involves a more complex, time-consuming, and costly multi-step process including cell extraction, modification in a lab, and reintroduction. |
In vivo therapy is a simpler process directly delivering new genes into target cells within the body, without cell extraction or manipulation. |
|
Control and precision |
|
|
Ex vivo therapy allows for greater control and verification of genetic modifications before cells are reintroduced into the patient, making it potentially safer and more precise. |
In vivo therapy offers less control over the modification process within the body. The immune system may react to viral vectors used in delivery, affecting treatment effectiveness. |
|
Application scope |
|
|
Ex vivo therapy is particularly useful for modifying blood or immune cells. |
In vivo therapy is typically used when treating a genetic disorder that affects one specific gene or when targeting an internal organ such as the heart, brain, or lungs |
Both delivery methods have their trade-offs, and the choice of delivery mechanism depends on the specific condition being treated. You can view the list of approved therapies here:
NEWDIGS FoCUS also maintains a pipeline analysis tracking the pipeline of products in development and FDA approvals of CGTs. The Pipeline Deep Dive shares greater detail on therapies in the pipeline. While clinical trial outcomes and regulatory approvals are never guaranteed, by 2026 we expect the following types of products may be available to patients:
- Oncology
- Additional therapies for B-cell leukemia and lymphoma
- Rare disease treatments
- Hematological conditions
- Additional treatments for ophthalmological conditions
- Neurological conditions
- Higher-prevalence disease treatments
- Age-related wet macular degeneration
- Osteoarthritis (knee)