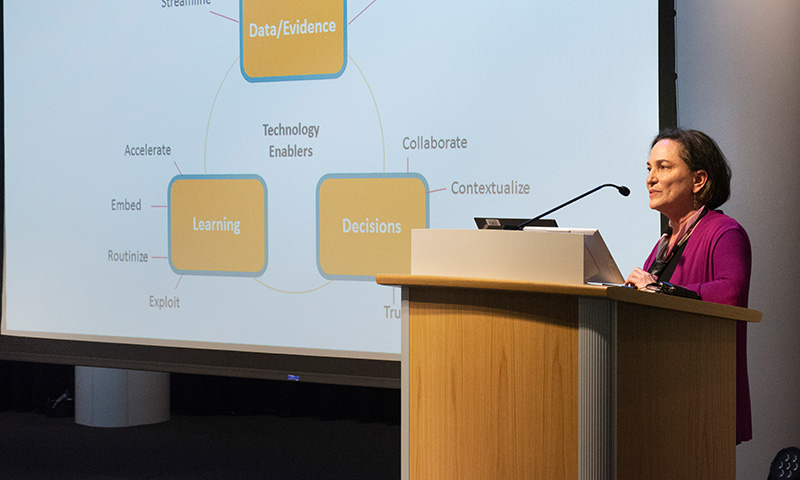
On June 11, 2019, MIT Center for Biomedical Innovation Executive Director Gigi Hirsch, MD, addressed the 4th Regulatory Innovation Evening held at Sanofi. This event brings pharmaceutical and regulatory professionals together to discuss advances and innovation in the industry. Dr. Hirsch was the keynote speaker and described the Dynamic Dossier in the Cloud project. The new initiative in the Center’s NEWDIGS consortium aims to develop a secure, cloud-based platform for advanced tools so pharmaceutical developers and regulators can seamlessly exchange and analyze data in real time.
Dr Hirsch explained how the Dynamic Dossier can use emerging digital tools, including Big Data analysis and encryption technologies for information security, to advance patient-centered innovation. Increasingly, she noted, “decisions need to be made collaboratively, not in our traditional silos,” in order to serve patients better. The Dynamic Dossier would provide an environment where data are shared appropriately and safely, decisions can be made in more transparent and timely ways, and learning that improves patient-centered decision making can be accelerated and more fully exploited.
Though the project is just getting started, Dr Hirsch underscored the importance of driving it toward real world application and impact. Two provisional pilot areas, she shared, where the Dynamic Dossier system will be immediately useful are in pharmacovigilance and mid- and long-term outcomes for new curative gene therapies.
After her address, Dr Hirsch and members of the audience, which included personnel from other regional companies in addition to those from Sanofi, discussed the potential value of the platform for ongoing benefit-risk analysis, design and implementation challenges in various therapeutic areas, and the opportunity to enable a more adaptive regulatory framework.
About NEWDIGS
The NEWDrug Development ParadIGmS(NEWDIGS) Initiative, led by the MIT Center for Biomedical Innovation, is an international “think and do tank” dedicated to delivering more value faster to patients, in ways that work for all stakeholders. NEWDIGS designs, evaluates and initiates advancements that are too complex and cross-cutting to be addressed by a single organization or market sector. Its members include global leaders from patient advocacy, payer organizations, biopharmaceutical companies, regulatory agencies, clinical care, academic research, and investment firms. For more information, visit http://newdigs.mit.edu.