Product profile challenges of CGT
 While any therapy may potentially have financial and administrative challenges, the profiles of products in the category of cell and gene therapies (CGTs) varies, affecting a need for innovative precision financing solutions.
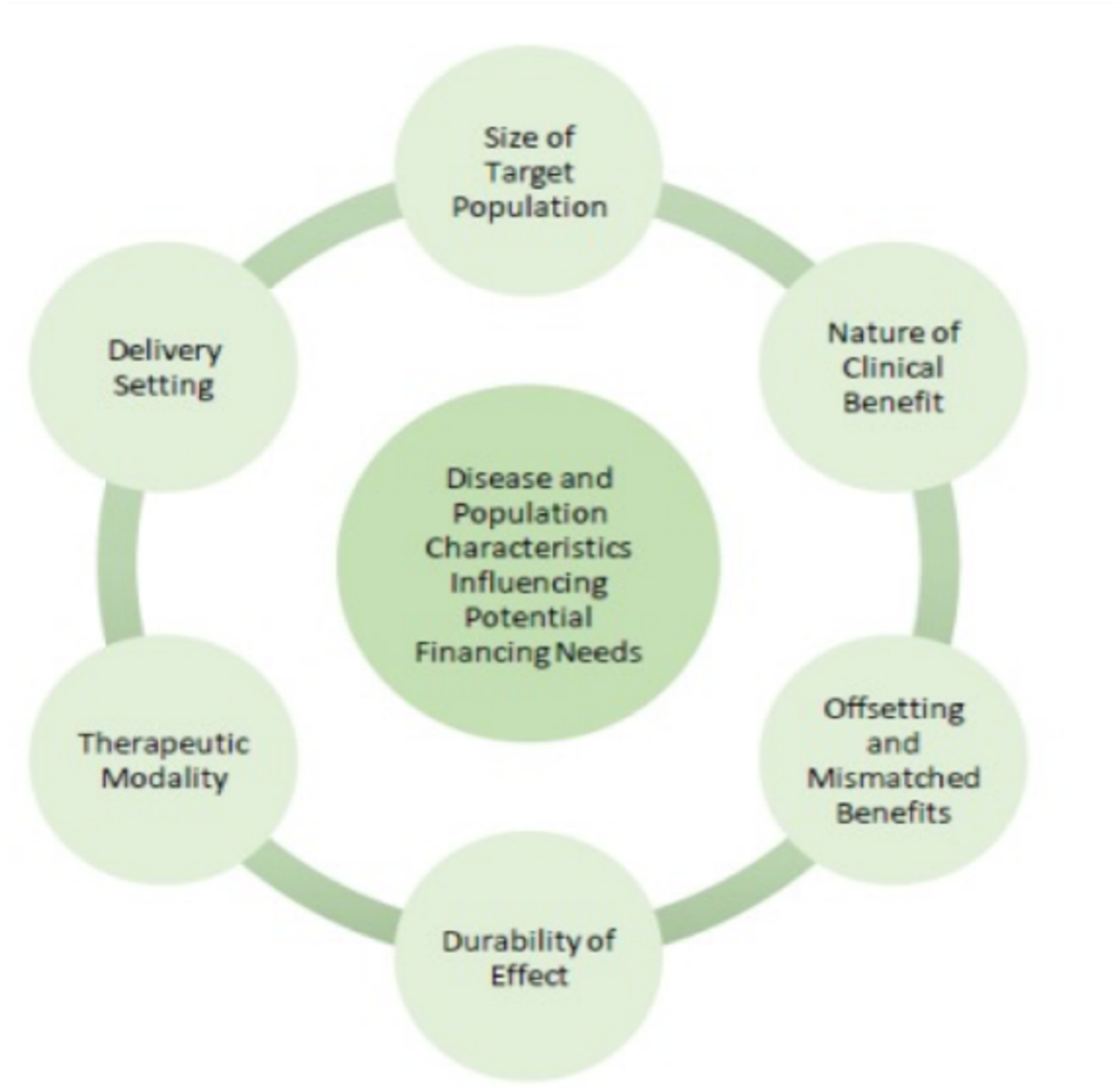
While any therapy may potentially have financial and administrative challenges, the profiles of products in the category of cell and gene therapies (CGTs) varies, affecting a need for innovative precision financing solutions.
Size of target population
The incidence and prevalence of each therapy’s target population will vary, affecting total treatment expenditures. Incidence refers to the number of new patients with a condition targeted by cell or gene therapy in a given year. This term could also refer to the annual number of patients reaching the point of disease progression, or the disease tipping point, where the patient becomes treatment eligible for cell or gene therapy. For example, a patient with a cancer diagnosis refractory to other treatments would be at the ‘tipping point’ for a CAR-T therapy indication and would be included in the incidence rate for that cell therapy. Prevalence refers to the total population with a particular condition. Some conditions only have an incident population due to high mortality rates, while a large prevalent population could mean that a large number of patients are waiting for a transformative treatment. While we do not know what therapy uptake will be, a significant prevalent population could increase the annual total cost of treatment in the short-term until a condition again only has an annual incident population needing treatment.
Nature of clinical benefit
For the eligible population, CGT will vary in their clinical benefit relative to existing standards of care across multiple dimensions, including:
- Expected benefit could range from a ‘cure’ that eliminates all symptoms and modifies all condition processes, to modest reversal or slowdown of selected disease processes.
- The likelihood and severity of adverse events.
- Patients do not all respond to treatment the same way and to the same extent. There may be non-responders, partial responders, and complete responders and that mix may vary by therapy.
- The clinical benefit compared with alternative treatment options.
Offsetting and mismatched benefits
CGTs will vary in their net financial impact to the healthcare system, to society, and to patients and their caregivers. Some therapies may reduce other healthcare treatment and service spending, while also generating higher patient benefit. Other therapies (for conditions that generate relatively low costs due to rapid death or absence of treatments for chronic morbidity), reduction may increase overall healthcare spending while generating patient benefits. Those patient benefits may reduce other social program costs and create additional economic benefits.
In addition, the timing of financial impact may vary; some treatments could result in immediate healthcare savings, while others could save money over time. For those therapies that save money over time, there could be a mismatch between who pays for the therapy and who reaps the benefits of the patient having received that therapy, as patients may switch payers over time.
Durability of effect
Efficacy duration will vary among patients for a single therapy and across therapies in general. Some may experience a lifetime of benefit, but many patients may not. In addition, the full duration of the effect for a particular treatment will not be known at the time of initial regulatory approval and launch, due to the urgent need in some cases of getting the treatment to the patients. Studies to determine duration of treatment efficacy are ongoing.
Therapeutic modality
Some CGTs employ viral vector delivery of genetic material in vivo while others are similar to advanced transplantation with cellular harvest, ex vivo transfection, and expansion, followed by cellular re-introduction. Therapeutic modality affects the delivery setting for treatment, payer benefit classification, provider reimbursement mechanisms, and patient financial participation, which in turn affects the financial challenges these therapies generate.
Delivery setting
CGTs vary in where the therapy is administered. Products may be administered within the inpatient or outpatient setting and at a limited number of Centers of Excellence or a broader set of facilities with established expertise. Some therapies may require extended hospital stays or intensive follow-up, while others may warrant only periodic clinic follow-up.
These characteristics not only affect the stakeholders requiring financial solutions but also affect ecosystem-related operational enablers such as appropriate provider networks, certifications, care coordination, product distribution, and data monitoring processes to ensure patients have access or financial solutions can be administered or both.
